Harris Health Moves Forward Proposal to Acquire Park Land for Expansion of Ben Taub Hospital
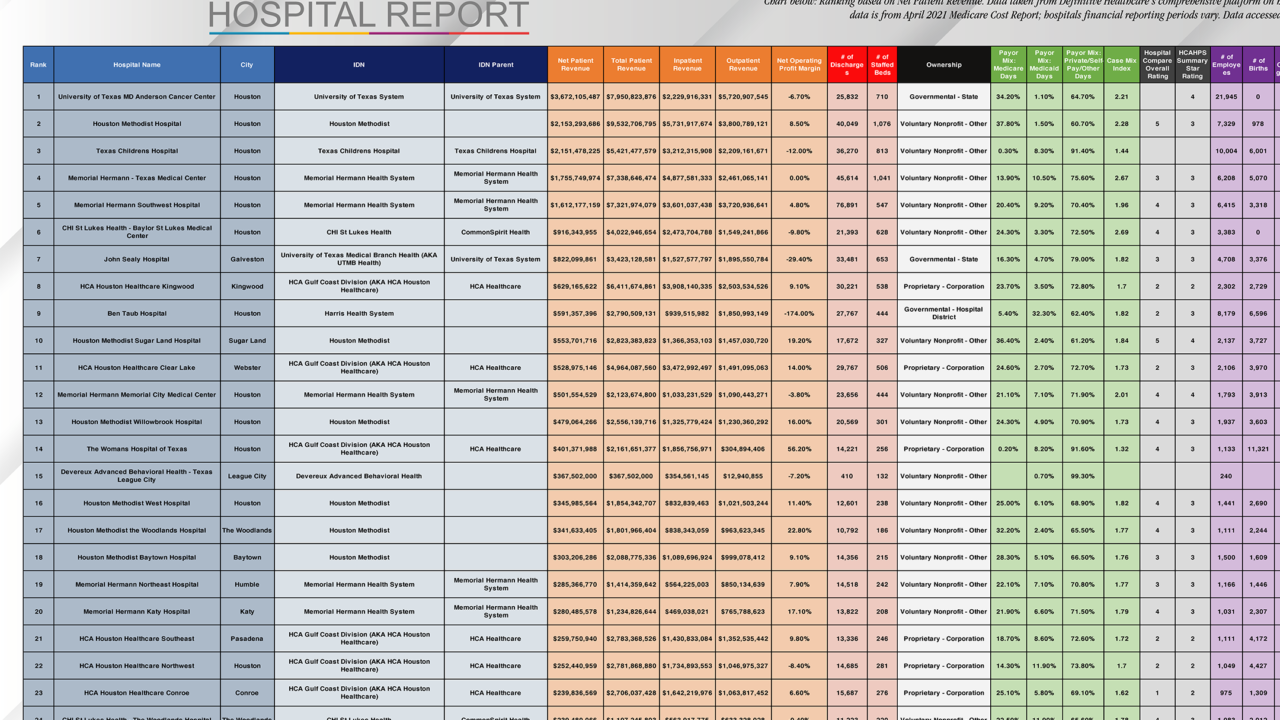
The Harris Health Board of Trustees unanimously adopted a resolution calling for the acquisition of land to support the critically needed expansion of Harris Health Ben Taub Hospital. Ben Taub Hospital has been consistently operating at and beyond its 402-bed capacity, and the need for its healthcare services is expected to grow at a rapid pace in the coming months. With cuts to federal Medicaid funding and insurance subsidies for lower-income individuals who purchase...