BY Jacque K. Steelman and Michael R. Alexander, Brown & Fortunato, P.C.
BY Jacque K. Steelman and Michael R. Alexander, Brown & Fortunato, P.C.
On April 29, 2024, Philips Respironics (“Philips”), a leading sleep apnea and respiratory therapy equipment manufacturer, announced that it reached a $1.1 billion settlement following allegations regarding faulty sleep machines. The settlement came from a class-action lawsuit against the company, claiming that certain devices were defective and potentially harmful to users. This legal action had wide-ranging implications for Philips and the millions of individuals relying on its equipment for managing sleep disorders and respiratory conditions. Philips did not admit any fault to the claims, and the agreement was made to resolve any uncertainty. This settlement follows a series of other legal actions taken against Philips this year.
On April 4, 2024, the Department of Justice of the United States Food and Drug Administration (“FDA”) filed a Complaint for Permanent Injunction on behalf of the United States. It alleged that one of the FDA’s inspectors discovered multiple violations at Philips’ Murrysville, Pennsylvania facility in which Philips was not complying with the Federal Food, Drug, and Cosmetic Act. Philips was then ordered by the United States  District Court to stop manufacturing and distributing most of its sleep and respiratory devices until it took specific measures to ensure an increase in the safety of its devices and ensure compliance with the Federal Food, Drug, and Cosmetic Act.
District Court to stop manufacturing and distributing most of its sleep and respiratory devices until it took specific measures to ensure an increase in the safety of its devices and ensure compliance with the Federal Food, Drug, and Cosmetic Act.
Philips entered into a Consent Decree of Permanent Injunction (“Decree”) with the United States on April 9, 2024, which involved the voluntary recall. The Decree provided a roadmap on actions and deliverables for Philips to meet to comply with FDA requirements. Under the decree Philips must retain outside experts to evaluate their facilities and ensure they are complying with all laws and regulations regarding device manufacturing. After five years of demonstrating their compliance with the Decree, Philips will then be able to petition the court to be relieved of the requirements under the Decree.
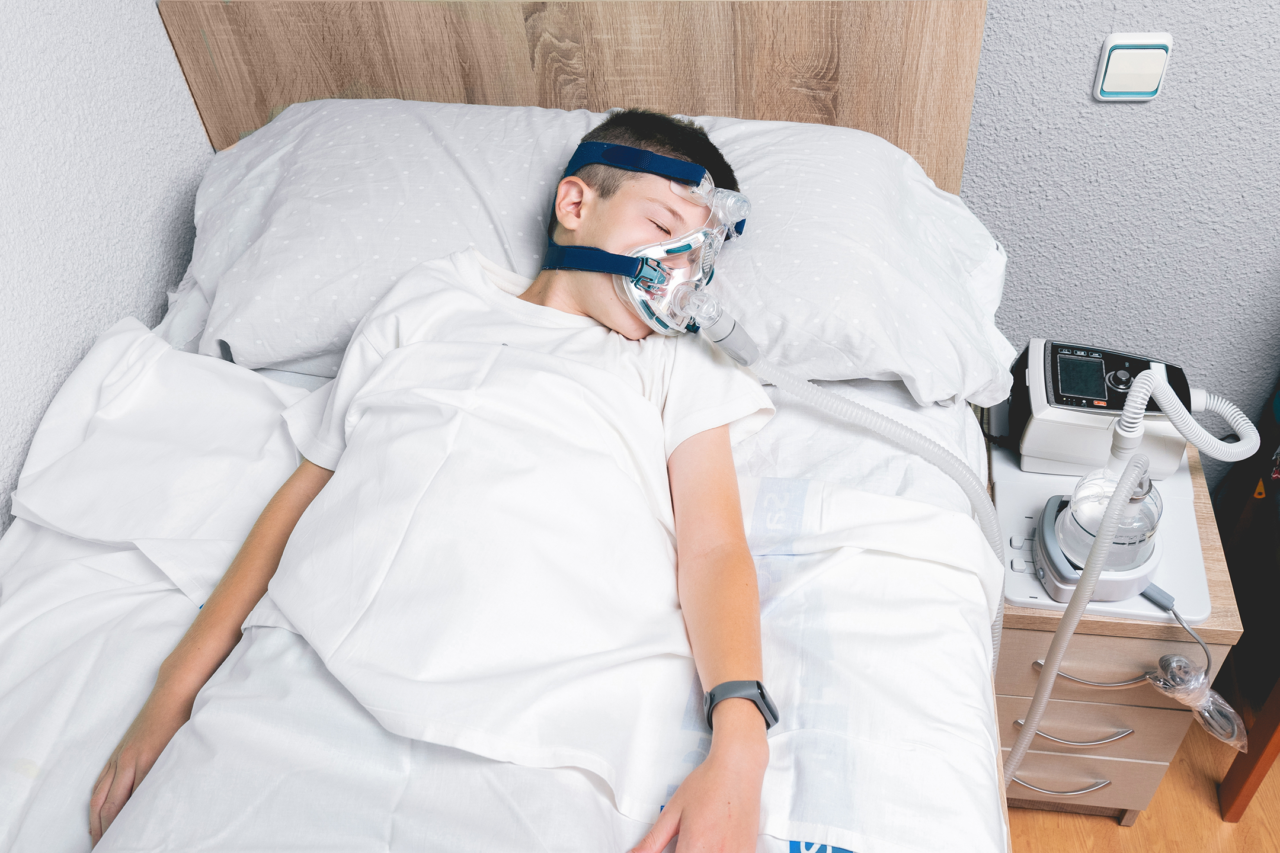
The overall issue stemmed from foam component in some of Philips’ sleep apnea machines and ventilators. This foam, known as polyester-based polyurethane (PE-PUR), was utilized in the sound abatement foam found in certain Continuous Positive Airway Pressure (CPAP) models, BiPAP, and mechanical ventilator devices. Over time, it was found that this foam could degrade and break down into particles that could be inhaled or ingested by users, potentially causing respiratory irritation, inflammation, and other adverse health effects.
The defect affected users worldwide who relied on these devices. Concerns escalated as reports surfaced linking the degraded foam to health issues such as respiratory infections, asthma exacerbations, and even reports of cancer. In response to mounting pressure from regulators, healthcare professionals, and affected individuals, Philips Respironics initiated a voluntary recall of affected devices in June 2021. This recall encompassed various models, affecting a significant portion of the company’s product lineup.
The recall impacted users dependent on these devices for managing sleep apnea and other respiratory conditions. Many individuals faced disruptions in their therapy routines, uncertainty regarding replacement options, and concerns about the potential health risks associated with continued use of affected equipment. This situation also placed a strain on healthcare providers and suppliers tasked with managing the logistics of device replacements and ensuring continuity of care for patients, further highlighting the gravity of the issue.
Following the recall, a class-action lawsuit was filed against Philips Respironics, alleging negligence, failure to warn, and other legal violations related to the defective devices. The lawsuit sought compensation for affected individuals, including reimbursement for the cost of their devices, medical expenses incurred due to related health issues, and damages for pain and suffering caused by defective products. Additionally, the lawsuit aimed to hold Philips Respironics accountable for its actions and compel the company to implement measures to prevent similar incidents.
After months of negotiations, Philips settled with the plaintiffs in the class-action lawsuit. The settlement represented a significant step towards resolving the legal claims and providing relief to affected individuals. Under the settlement terms, Philips agreed to establish a fund to compensate eligible claimants for their losses and expenses related to the defective devices. This fund would cover costs such as device replacements, and other associated expenses incurred by affected individuals. Within the settlement is an additional $25 million to pay for medical monitoring for patients who will still need to be evaluated for years to come to determine whether there are further illnesses caused by the foam.
The settlement outlined measures aimed at addressing the broader implications of the recall and preventing similar incidents from occurring in the future. Philips is committed to enhancing its quality control processes, improving transparency and communication with regulators and consumers, and implementing safeguards to promptly identify and address potential safety issues. These measures were designed to rebuild trust in the company’s products and restore confidence among users and healthcare professionals, demonstrating Philips’ commitment to product safety.