The world’s population has increased by more than 2.6-fold in the last 60 years. The growing trend continues – projections indicate that the number of people living on our planet will grow to 9 billion by 2037 from 8 billion in 2022. These numbers underscore the need for considering family planning; however, there have been limited breakthroughs in contraception in recent decades. Specifically for men, there are no oral contraceptive pills available.
In a study published in the journal Science, researchers at Baylor College of Medicine and collaborating institutions show in animal models that a novel, non-hormonal sperm-specific approach offers a promising option for reversible human male contraception.
“Although researchers have been investigating several strategies to develop male contraceptives, we still do not have a birth control pill for men,” said corresponding author Dr. Martin Matzuk, director of the Center for Drug Discovery and chair of the Department of Pathology and Immunology at Baylor. “In this study we focused on a novel approach – identifying a small molecule that would inhibit serine/threonine kinase 33 (STK33), a protein that is specifically required for fertility in both men and mice.”
Previous research has shown that STK33 is enriched in the testis and is specifically required for the formation of functional sperm. In mice, knocking out the Stk33 gene renders the mice sterile due to abnormal sperm and poor sperm motility. In men, having a mutation in the STK33 gene leads to infertility caused by the same sperm defects found in the Stk33 knockout mice. Most importantly, mice and men with these mutations have no other defects and even have normal testis size.
“STK33 is therefore considered a viable target with minimal safety concerns for contraception in men,” said Matzuk, who has been on faculty at Baylor for 30 years and is Baylor’s Stuart A. Wallace Chair and Robert L. Moody, Sr. Chair of Pathology and Immunology. “STK33 inhibitors have been described but none are STK33-specific or potent for chemically disrupting STK33 function in living organisms.”
Finding an effective STK33 inhibitor
“We used DNA-Encoded Chemistry Technology (DEC-Tec) to screen our multi-billion compound collection to discover potent STK33 inhibitors,” said first author Dr. Angela Ku, staff scientist in the Matzuk lab. “Our group and others have used this approach before to uncover potent and selective kinase inhibitors.”
The researchers uncovered potent STK33-specific inhibitors, from which they successfully generated modified versions to make them more stable, potent and selective. “Among these modified versions, compound CDD-2807 turned out to be the most effective,” Ku said.
“Next, we tested the efficacy of CDD-2807 in our mouse model,” said co-author Dr. Courtney M. Sutton, postdoctoral fellow in the Matzuk lab. “We evaluated several doses and treatment schedules and then determined sperm motility and number in the mice as well as their ability to fertilize females.”
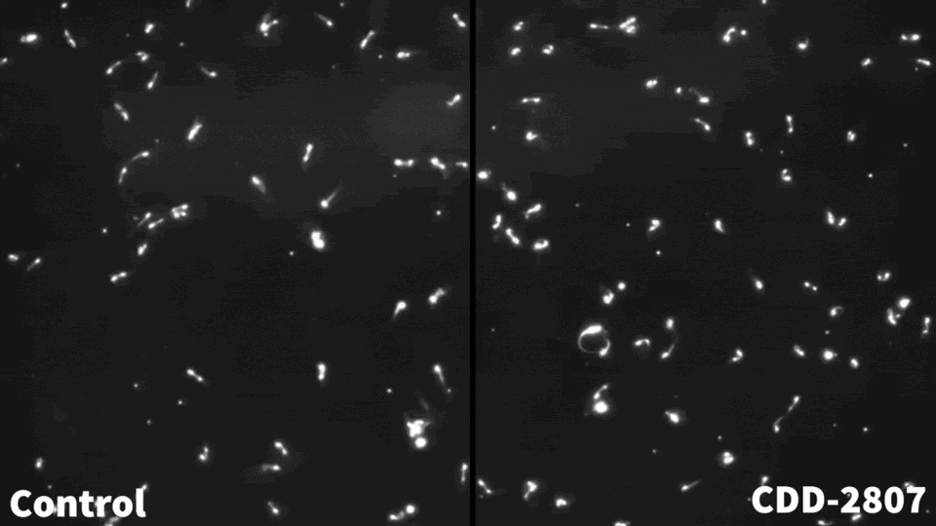
Microscopic image showing the effects of compound CDD-2807 on sperm motility.
Compound CDD-2807 effectively crossed the blood-testis barrier and reduced sperm motility and numbers and mice fertility at low doses. “We were pleased to see that the mice did not show signs of toxicity from CDD-2807 treatment, that the compound did not accumulate in the brain, and that the treatment did not alter testis size, similar to the Stk33 knockout mice and the men with the STK33 mutation,” Sutton said. “Importantly, the contraceptive effect was reversible. After a period without compound CDD-2807, the mice recovered sperm motility and numbers and were fertile again.”
“In our paper, we also present the first crystal structure for STK33,” said co-author Dr. Choel Kim, associate professor of biochemistry and molecular pharmacology and member of the Dan L Duncan Comprehensive Cancer Center at Baylor. “Our crystal structure showed how one of our potent inhibitors interacts with STK33 kinase in three dimensions. This enabled us to model and design our final compound, CDD-2807, for better drug-like properties.”
“This study was a tour de force by our team in the Center for Drug Discovery at Baylor and our collaborators,” said co-author Dr. Mingxing Teng, assistant professor of pathology and immunology and of biochemistry and molecular pharmacology at Baylor. Teng also is a Cancer Prevention Research Institute of Texas Scholar and a member of the Dan L Duncan Comprehensive Cancer Center at Baylor. “Starting with a genetically validated contraceptive target, we were able to show that STK33 is also a chemically validated contraceptive target.”
“In the next few years, our goal is to further evaluate this STK33 inhibitor and compounds similar to CDD-2807 in primates to determine their effectiveness as reversible male contraceptives,” Matzuk said.
Additional co-authors of the paper affiliated with Baylor College of Medicine are Kiran L. Sharma, Hai Minh Ta, Kurt M. Bohren, Yong Wang, Srinivas Chamakuri, Ruihong Chen, John M. Hakenjos, Ravikumar Jimmidi, Katarzyna Kent, Feng Li, Jian-Yuan Li, Lang Ma, Chandrashekhar Madasu, Murugesan Palaniappan, Stephen S. Palmer, Xuan Qin, Zhi Tan, Yasmin M. Vasquez, Jian Wang, Zhifeng Yu, Qiuji Ye and Damian W. Young. Co-authors Matthew B. Robers and Jennifer Wilkinson are affiliated with Promega Corp., and Banumathi Sankaran is affiliated with Lawrence Berkeley National Laboratory.